-
From the Society for Clinical Vascular Surgery
Outcomes after retroflexed gracilis muscle flap for vascular infections in the groin
Ahsan T. Ali, MD,a Mario Rueda, MD,b Sarasijhaa Desikan, MD,a Mohammed M. Moursi, MD,a Ruosu An, MD,a Horace Spencer, MS,a Steven Rueda, MD,c and John F. Eidt, MD,d Little Rock, Ark; Baltimore, Md; Cleveland, Ohio; and Dallas, Tex
Objective: Multiple catheterizations and procedures on the femoral arteries can increase the risk of infection and eventual destruction of the overlying skin and subcutaneous tissue. Without adequate tissue coverage, vascular structures are exposed and, thus, vulnerable to disruption. This can lead to loss of limb and/or life and carries a significant mortality. We hypothesized that gracilis muscle flap (GMF) was a reliable adjunct in providing healthy tissue coverage for a complex surgical problem.
Methods: Retrospective review of charts was performed on all patients who had undergone GMF for groin infections at a tertiary care medical center.
Results: From 1997 to 2012, GMF was performed in 68 limbs (64 patients) by vascular surgeons for infectious etiology to cover the common femoral artery. At the time the GMF was placed, the femoral artery had synthetic graft/patch in 14 limbs, whereas 54 limbs had procedures with autologous conduit. Complete healing was achieved in 58 (85%) limbs. Treatment was deemed not successful in 10 limbs where patients continued to have persistent infection. Six out of 10 limbs had anastomosis disruption requiring emergent ligation of the common femoral artery. Nine patients died during the perioperative period (30-day). There were a total of 13 amputations in 12 patients. Limb salvage was achieved in 55 limbs (81%). Univariate analysis suggested that patients that had revascularization procedures with synthetic graft had a higher complication rate compared with autologous/vein reconstruction (24% vs 5%; P [ .021). This group also has a higher rate of persistent infection compared with the autologous group (24% vs 2%; P [ .006). Patients older than 75 years at the time of GMF had a higher incidence of GMF-related complications (57% vs 5%; P [ .04). Multivariate analysis confirmed that presence of prosthesis led to higher incidence treatment failures and muscle flap complications at the surgical site (odds ratio, 6.6; P [ .04; and odds ratio, 13.3; P [ .03, respectively).
Conclusions: GMF is technically simple to perform and provides durable soft tissue coverage with a high rate of healing for complex groin wounds even in the presence of synthetic conduit. (J Vasc Surg 2016;64:452-7.)
Dealing with a vascular infection in the groin can be very challenging, especially if there is destruction of overlying skin and soft tissue.1-4 The most frequent site for catheter-based interventions is the femoral artery.1-5 The femoral area is also the most common site of revascularization procedures for open bypasses. Up to 7% of the patients may develop infections when having procedures in the femoral vessels, as opposed to 0.5% at more proximal
From the Division of Vascular Surgery, Department of Surgery, University of Arkansas for Medical Sciences, Little Rocka ; the Department of Surgery, Johns Hopkins University School of Medicine, Baltimoreb ; the Department of Plastic Surgery, Cleveland Clinic Foundation, Clevelandc; and the Department of Surgery, Baylor Heart and Vascular Hospital, Dallas.d
Author conflict of interest: none.
Presented at the Fortieth Annual Meeting of the Society for Clinical Vascular Surgery, Las Vegas, Nev, March 12-14, 2012.
Correspondence: Ahsan T. Ali, MD, Division of Vascular Surgery, Department of Surgery, University of Arkansas for Medical Sciences, 4301 West Markham St, Ste 520-2, Little Rock, AR 72205 (e-mail: sibiahsan@ yahoo.com).
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest. 0741-5214
Copyright 2016 by the Society for Vascular Surgery. Published by Elsevier Inc.
http://dx.doi.org/10.1016/j.jvs.2016.03.010sites.1-4 Factors that can increase risk are reoperative surgery, poor hygiene, postoperative bleeding, poor nutrition, and systemic infections from central line or urinary tract.3-6 Other comorbidities such as obesity and end-stage renal disease are strong predictors for graft infections and/or surgical site infection.3-7 The presentation can vary from deep soft tissue infection with necrosis of the overlying skin to limb or life-threatening sepsis with hemorrhage.4-6 The ideal treatment would be to excise all involved prosthetic while maintaining vascular continuity using an autologous conduit.6,7 However, the overlying skin and soft tissue has to be healthy and substantive enough to be able to provide adequate coverage for the underlying vascular structures. Muscle flaps have traditionally been used as an adjunct to provide coverage and assist in treating local infections.8-10 We reviewed our experience in the usage of gracilis muscle flap (GMF) as an adjunct in the treatment of graft infection with associated groin breakdown.
METHODS
This study was undertaken at The University of Arkansas for Medical Sciences hospital and the Central Arkansas Veterans Hospital. The institutional review boards approved this study. According to the policy and procedures required by the institutional review boards, informed consent was not required. This was a retrospective chart
JOURNAL OF VASCULAR SURGERY
August 2016Ali et al
1
review. Patients who underwent vascular procedures and subsequently had GMF between 1997 and 2012 were identified.
Basic demographics, initial vascular procedure as well as type of graft (autologous vein vs synthetic) were recorded. A successful treatment was defined as resolution of infection per limb treated, with healing of overlying skin and soft tissue and no requirements for long-term antibiotics. Treatment failure was defined as persistent infection leading to disruption of the vascular anastomosis or failure to heal. Patients undergoing GMF for indications other than infection were excluded. Primary outcome assessed was freedom from infection with complete healing. Secondary outcomes were limb salvage, GMF-related complications, and long-term survival. Comparison between synthetic and autologous material (vein) was also performed using univariate and multivariate analysis for predictors of poor outcome.
Surgical technique. All GMF procedures were performed by vascular surgeons. A longitudinal medial thigh incision was made avoiding injury to the great saphenous vein (GSV). If the GSV had been recently harvested, then the same incision was used. The gracilis muscle was identified and its insertion tendon isolated. In order to identify the gracilis the following points were considered.
The muscle is tendinous in the distal third, and there are no perforating branches in the distal one-half of the gracilis muscle body. It also tapers down to a tendon, whereas the sartorius muscle maintains its rectangular shape. This helps differentiate the gracilis from the sartorius, which has a segmental blood supply from the superficial femoral artery (SFA).10 The surgeon’s index finger is easily passed circumferentially around the muscle. This is an essential step required for mobilization. The tendon is then retracted proximally and disconnected as distally as possible. It is important to mobilize and dissect the adductor longus muscle on both sides. No nerve structure should be found anterior to the gracilis muscle.11 The muscle is then retroflexed back proximally to cover the femoral vessels. We do not pass it through the adductor longus muscle or detach it from its proximal attachment to the pubis. Furthermore, we do not intentionally takedown the medial circumflex artery branch. The GMF is then secured with 2-3 absorbable sutures to the inguinal ligament and surrounding structures.
This technique is a variation from Morasch et al8 in which they had mobilized the gracilis muscle from the pubic bone and passed through the adductor longus muscle. The technique video is available on YouTube: https://www.youtube.com/watch?v¼FngQbkHtrrE.
Statistics. Statistical analysis was performed using Stata 11.0 (Stata Corp LP, College Station, Tex). The c2 or Fisher exact tests were used for categoric variables and for variables in which frequency was limited to 5 or less occurrences per subgroup. P value required for inclusion in the multivariate analysis was <.05. Data were reported as mean 6 standard deviation. Multivariate analysis using logistic regression was used to assess predictors of primary and secondary end points. Odds ratios were obtained.
RESULTS
During a 15-year period (1997-2012), 68 limbs (64 patients) had GMF for coverage of the ipsilateral femoral artery for an infectious etiology. The mean age was 64.2 +/- 8.1 years. Majority were men (n ¼ 48). The most common presentation was a nonhealing groin wound or a sinus tract in 32/68 limbs (47%). Clinical presentations are mentioned in detail in Table I.
There were 10 patients who were taken to the surgery emergently for ruptured femoral artery aneurysm/pseudoaneurysm or hemorrhage from the femoral artery (Table I). The original vascular procedures are also listed in Table I. The mean follow-up was 34 +/- 28 months (mean 6 standard deviation). The mean number of procedures on each patient was 2.5 +/- 1.2.
Outcomes are described in Table II. Repair of the common femoral artery was undertaken with autologous
Table I.Initial presentation, original procedures
Demographics
Number of patients
64
Number of limbs treated
68
Mean age, years
64.2 6 8.2
Men:women
48:16
Original procedures
Aortofemoral bypass graft
15
Femoral popliteal bypass
17
PTFE
11
Vein/Cryovein
6
Common femoral artery endarterectomy
9
Repair femoral artery from catheter injury
5
Femoral-femoral artery bypass/revision
4
Cut down for endovascular aneurysm repair
2
Embolectomy
3
Femoral artery pseudoaneurysm repair
11
Clinical presentation
Nonhealing wound/exposed femoral artery
32
FAP with intact skin
12
Ischemia/occult infection without FAP
8
FAP with sinus tract
4
Ruptured FAP or expanding hematoma
10
FAP, Femoral artery aneurysm/pseudoaneurysm; PTFE, polytetrafluoroethylene
Table II.Outcomes/gracilis muscle flap (GMF)-related complications
Results
Successful treatment (limbs)
58
Persistent infection or treatment failure (limbs)
10
All amputations
13 (5 þ 9)
In-hospital death
9
Complications from muscle transfer
Necrosis of flap
3
Hematoma
3
Seroma
1
JOURNAL OF VASCULAR SURGERY
August 2016Ali et al
2
Table III. Treatment failure with persistent infection
Age/sex
Original procedure
Presentation
Procedure with GMF
Conduit
Microbiology
Complications
Graft salvage
Outcomes
79/M
Common femoral endarterectomy
Nonhealing incision
Debridement of wound
Synthetic pericardium
Pseudomonas aeruginosa
Persistent infection
No
Disruption of anastomosis, amputation/death
87/M
AFBG
Infected FAP
Replacement of distal limbs with rifa mpin soaked graft
Dacron
Never cultured
Persistent infection/ sepsis
N/A
MSOF/death
67/M
Direct repair of the CFA after catheterrelated hematoma
Non healing incision
Debridement
Direct repair
Staphylococcus aureus, Enterococcus faecalis
Necrosis of flap, multiple abscesses
N/A
Multiple I&D with VAC dressing
84/M
Fem-distal with vein
Bleeding from anastomosis
Repair bovine pericardium
Bovine pericardium
Never cultured
Sepsis
Yes
Death
49/M
Fem-pop with vein
Hematoma requiring evac
Debridement
Vein
Never cultured
Local abscess
Yes
Multiple I&D, evacuation of hematoma
84/M
Infected AFBG limb
Infected AFBG limb
Replace limb with Dacron
Synth
Never cultured
Persistent infection
No
Death while undergoing
58/M
Fem-fem graft revision
FAP
Removal of fem-fem,
Auto
Streptococcus viridans
Anastomosis disruption
No
BKA
70/M
Fem-pop with vein
Bleeding from anastomosis
Primary repair with
Auto
No cultures
Anastomosis disruption
No
AKA
54/F
Fem-pop with PTFE previous AFBG
FAP/bleeding
Ligation of fem-pop/ AFBG
Bovine pericardium
Pseudomonas
Disruption with bleeding
No
Ligation of limb > hip disarticulation
60/M
AFBG
Infected FAP, bilateral
NAIS
Deep vein
MRSA
Anastomotic disruption
No
AKA, MSOF death
AFBG, Aortofemoral bypass graft; AKA, above knee amputation; BKA, below knee amputation; F, female; FAP, femoral artery aneurysm/pseudoaneurysm; fem-fem, femoral-femoral; fem-pop, femoral popliteal; GMF, gracilis muscle flap; I&D, incision and drainage; M, male; MRSA, methicillin-resistant Staphylococcus aureus; MSOF, multisystem organ failure; N/A, not applicable; NAIS, neo-aortic iliac system; VAC, vacuum-assisted device.
material at the time of GMF coverage in 54 limbs, whereas 14 limbs had synthetic graft material. Primary outcome was complete healing of the overlying skin and soft tissue. This was achieved in 58 of the 68 limbs. However, in 10 patients, there was evidence of persistent infection and thus were considered treatment failures. These patients are described in detail in Table III. In six of the 10, there was acute hemorrhage, and the common femoral artery had to be ligated, resulting in a major amputation in five patients. There were an additional eight major amputations in eight patients bringing the total number of amputations to 13. These are described in Table IV. These patients either had no revascularization options (lack of conduit or target) or had nonviable limb on initial presentation. Our primary objective in these patients was to save life over limb by eradicating the infected prosthesis and covering the exposed femoral vessels.
The number of in-hospital or 30-day deaths occurred in nine out of 64 patients. Five out of the nine patients died in the treatment failure group with persistent infection (Table III). As mentioned earlier, five of these patients had the femoral artery ligated with a major amputation.
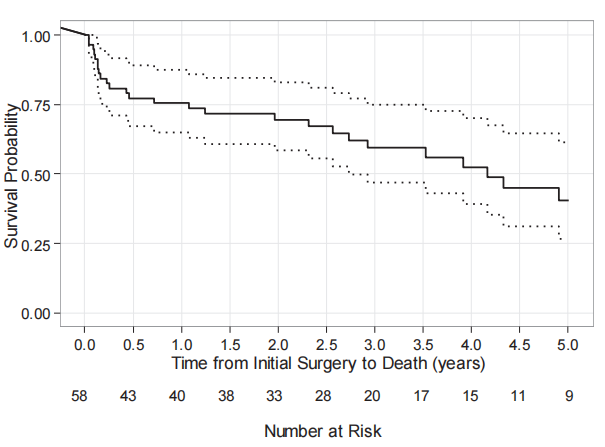
An additional four patients died in the postoperative period and have been described in Table V. They had apparent successful eradication of infection and vascular reconstruction, yet died from other causes (Table V). The overall survival for the entire group was typical of vascular patients (Fig).
From surgical specimen with positive yield, 46% had a single organism while 25% had polymicrobial growth. There were no results available in 14/68 limbs (20%) and no organisms identified in 9%.
Univariate analysis. Comparing autologous vs synthetic graft, the autologous graft was associated with a lower rate of persistent infection or treatment failure. The rate of failure was almost 12 times higher in the synthetic group (24% vs 2%) in the autologous reconstructions, which was highly significant (P ¼ .006). There were no significant differences in flap specific complications across the synthetic and autologous tissue reconstruction groups (P ¼ .16). Complications related to GMF transfer are described in Table II. Univariate analysis for age demonstrated that flap complications rate was 57% in the older group (age >75 years) vs 5% in the younger cohort (P ¼ .04).
JOURNAL OF VASCULAR SURGERY
August 2016Ali et al
3
Table IV. Additional amputations (distal ischemia-related)
Age/sex
Original surgery
Presentation
Procedure with GMF
Conduit
Microbiology
Reason for amputation
Level
52/M
Fem-pop with PTFE
Acute occlusion, had thrombectomy but got infected with open groin
Removal of fem-pop, deep vein femoralprofunda bypass
Deep vein
MRSA, Porevotella
Ischemia, no conduit for distal bypass
BKA
64/F
Fem-pop
Graft thrombosis, nonviable limb
Removal of fem-pop w repair of CFA w
Vein
Peptostreptococus, Bacteriods
Non-viable limb
AKA
54/M
Fem-pop w PTFE
Sepsis
Removal of fem-pop, repair CFA
Vein
Staphylococcus aureus
Sepsis, hypotensive
AKA
56/M
Thrombosis of fem-pop
Infected fem-pop/acute ischemia
EIA to profunda bypass w vein GMF
Vein
No growth
No vein, poor run-off
BKA
50/M
Fem-fem
FAP, infected
Removal of fem-fem, patch repair with pericardium,
Bovine
Never cultured
Irreversible ischemia, no vein
BKA
58/M
AFBG/bilat fem-pop
Occult infection, occluded AFBG, fempop
Replace AFBG with NAIS, removal of fem-pop
Deep vein
Candida albicans
No conduits, no signal in foot
BKA
63/M
CFA end with bovine
Non healing wound
Repair with vein
Deep vein
VRE
Tissue loss with no conduit
AKA
63/M
Aortobiliac
Medial thigh abscess
Iliofemoral with vein
GSV
MSSA
Distal tissue loss, nonviable foot
BKA
AFBG, Aortofemoral bypass graft; AKA, above knee amputation; BKA, below knee amputation; CFA, common femoral artery; EIA, external iliac artery; F, female; FAP, femoral artery aneurysm/pseudoaneurysm; fem-fem, femoral-femoral; fem-pop, femoral popliteal; GMF, gracilis muscle flap; GSV, great saphenous vein; M, male; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, Methicillin sensitive Staphylococcus aureus; NAIS, neo-aortic iliac system; PTFE, polytetrafluoroethylene; VRE, vancomycin-resistant Enterococcus.
Table IV. Additional amputations (distal ischemia-related)
Age/sex
Original surgery
Presentation
Treatment
Conduit
Microbiology
Wound closure
Cause of death
54/M
AFGB/Ax-fem bypass
Acute ischemia, groin sinus
NAIS, bilateral SFA remote end
Deep vein
Enterococcus faecalis, Lactobacilus
VAC
Presumed MI 3 weeks after discharge
68/F
Fem-distal vein bypass 3 weeks before presentation
Acute hemorrhage from anastomosis
Repair with pericardial patch, GMF
Pericardial patch
Candida, klebsiella, Escherichia coli
VAC
Sepsis
99/F
Embolectomy 4 weeks before presentation
Infected FAP
Repair with vein patch, GMF
Vein
Pseudomonas aeruginosa
Primary closure
MSOF in hospital
71/F
Femoral endarterectomy
Infected FAP
Repair with deep vein
Vein
VRE, MRSA
VAC
MSOF in hospital
AFBG, Aortofemoral bypass graft; AX-fem, axillary-femoral; F, female; FAP, femoral artery aneurysm/pseudoaneurysm; fem, femoral; GMF, gracilis muscle flap; M, male; MI, myocardial infarction; MRSA, methicillin-resistant Staphylococcus aureus; MSOF, multisystem organ failure; NAIS, neo-aortic iliac system; SFA, superficial femoral artery; VAC, vacuum-assisted device; VRE, vancomycin-resistant Enterococcus.
Multivariate analysis. A multivariable analysis revealed the gracilis-related flap complications had a 6.6 times higher odds of developing a flap complication when a synthetic graft was used compared to the group with vein reconstruction (P ¼ .04). Likewise the frequency of persistent infection was 13.3 times higher when a synthetic vascular conduit/graft was used compared with autogenous (P ¼ .03).
DISCUSSION
Reoperative surgery and/or hematoma in the groin area after catheterizations could lead to an increased risk of infection.1-7 A nonhealing incision with exposed femoral
artery and active infection can be a nightmare for the vascular surgeon. The presence of a complex wound is not the only issue as these patients are often nutritionally depleted with significant other comorbidities.1,2,6 Even with an in-line bypass using autologous conduits, lack of adequate soft tissue coverage could render the femoral vessels exposed and prone to disruption. Restoring vascular continuity is the primary goal. However, covering the area with healthy soft tissue is an equally important adjunct for a successful outcome.12-14
An extra anatomical bypass may not be an option if the SFA is occluded with no outflow above the knee joint.
JOURNAL OF VASCULAR SURGERY
August 2016Ali et al
4
Fig. 1 Survival Kaplan-Meyer curve after discharge.
Muscle flaps have been used as an adjunct to treat groin infections.8-13 Well-perfused muscle tissue provides coverage to vascular structures and allows successful treatment of infection with soft tissue healing. The profunda femoris artery is usually patent and spared from catheterbased interventions. The gracilis muscle derives its blood supply from the branches of the deep profunda artery as a pedicle. It also has a greater arc of rotation and minimal restrictions in postoperative ambulation.11 This makes the GMF an appealing option for groin wound coverage. The use of sartorius muscle has been well described; however, we have found it often to be ischemic or inflamed from an invasive infection with an occluded SFA.10-12 The use of sartorius muscle is quite infrequent at our institute and, thus, was not included.
In the current study, the majority of limbs (58 out of 68) were successfully treated with complete resolution of the infection. These patients were able to be discharged without any additional surgical interventions and did not require long-term antibiotics. The maximum duration of antimicrobial treatment was 6 weeks for patients who survived and were discharged.
The type of graft used for revascularization prior to performing a GMF also affected the outcome. Synthetic grafts were more prone to developing infections. Univariate as well as multivariate analysis in our study suggested that there were acceptable cure rates for patients who underwent femoral artery reconstruction with a synthetic material. However, the rate of flap complications was significantly increased when compared to autologous conduit (24% vs 5%). Amputations in the group as a whole represent a cohort of patients with usually failed bypasses
and end-stage vascular disease. The primary goal was always “life over limb.” The authors believed that the groin with active infection without adequate tissue coverage could result in life-threatening hemorrhage even with an autologous repair. It is important to point out that a significant number of autologous vein reconstructions had been performed for infected synthetic bypasses/patches. The occurrence of treatment failures was significantly higher in patients with synthetic grafts compared with autologous graft or patch (P ¼ .03). The autologous graft material was ipsilateral GSV, femoral vein, translocated endarterectomized SFA, and cryo vein (two cases). Using a vein graft or patch rendered a favorable outcome with a decreased complication rate and a significantly higher freedom from infection.
Early studies have described the use of a GMF with favorable short-term results.8 However, to date this is the largest report of a GMF by vascular surgeons for complex groin wounds after vascular procedures.
We also observed that the highest frequency of treatment failure was in patients with a gracilis flap over synthetic graft with primary skin closure. The authors recommend using femoral vein, or when a suitable vein is not available, a translocated endarterectomized SFA can be used. With the use of GMF, we strongly recommend leaving overlying skin open and using local wound care and/or vacuum assisted devices (VACs). VACs have been used and recommended in reports for Szilagyi III wounds with acceptable results.15 Although VACs have shown promise, we recommend adjunctive usage of a GMF, especially when the vessels are exposed. Trying to primarily close the skin/soft tissue can place the wound under
JOURNAL OF VASCULAR SURGERY
August 2016Ali et al
5
significant tension. We do not recommend placing the sponge in VAC therapy directly in contact with the vascular structures. This study was limited as it was a retrospective review. Additional risk factors such as diabetes, smoking, body mass index/obesity, hypertension, and coronary artery disease were not factored in the final analysis. Also, we did not assess the previous sartorius muscle flap failures that eventually ended up with a GMF and synthetic graft material replaced with autogenous conduit. Finally, no other specialist was involved as vascular surgeons performed all GMF procedures. The procedure did not take longer than 15-20 minutes of additional time. Currently at our institution, we use the gracilis muscle retroflexed flap as the first line treatment when a muscle flap is planned to treat a complex groin wound.
CONCLUSIONS
Creation of the GMF can serve as an important adjunct for treating femoral artery infections while providing healthy soft tissue coverage. The cure rate after gracilis muscle rotational flap was significantly higher when vein graft or patch was used compared with synthetic grafts. Future research could focus on identifying patients that are at high risk and perform a gracilis flap prophylactically to prevent an infection.
AUTHOR CONTRIBUTIONS
Conception and design: AA, MR, MM, JE
Analysis and interpretation: AA, MR, SR, HS, JE
Data collection: AA, MR, SD, RA
Writing the article: AA, MR, JE
Critical revision of the article: AA, MR, JE
Final approval of the article: AA, MR, SD, MM, SR, HS, RA, JE
Statistical analysis: MR, SR, HS
Obtained funding: AA
Overall responsibility: AA
AA and MR contributed equally to this article and share
co-first authorship.REFERENCES
- Bunt TJ, Haynes JL. Synthetic vascular graft infection. The continuing headache. Am Surg 1984;50:43-8.
- Kearney RA, Eisen HJ, Wolf JE. Nonvalvular infections of the cardiovascular system. Ann Intern Med 1994;121:219-30.
- Jones L, Braithwaite BD, Davies B, Heather BP, Earnshaw JJ. Mechanism of late prosthetic vascular graft infection. Cardiovasc Surg 1997;5:486-9.
- Reiffel AJ, Henderson PW, Karwowski JK, Spector JA. An interdisciplinary approach to the prevention and treatment of groin wound complications after lower extremity revascularization. Ann Vasc Surg 2012;26:365-72.
- Kuy S, Dua A, Desai S, Dua A, Patel B, Tondravi N, et al. Surgical site infections after lower extremity revascularization procedures involving groin incisions. Ann Vasc Surg 2014;28:53-8.
- Ali AT, Bell C, Modrall JG, Valentine RJ, Clagett GP. Graft assoiciated hemorrhage from femoral-popliteal vein grafts. J Vasc Surg 2005;42: 667-72.
- Hepp W, Schulze T. The management of infected grafts in reconstructive vascular surgery. Thorac Cardiovasc Surg 1986;34:265-8.
- Morasch MD, Sam AD, Kibbe MR, Hijjawi J, Dumanian GA. Early results with use of gracilis muscle flap coverage of infected groin wounds after vascular surgery. J Vasc Surg 2004;39:1277-83.
- Mahoney J. Salvage of the infected groin vascular graft with muscle flaps. Ann Plast Surg 1989;22:252-6.
- Landry GJ, Carlson JR, Liem TK, Mitchell EL, Edwards JM, Moneta GL. The sartorius muscle flap: an important adjunct for complicated femoral wounds involving vascular grafts. Am J Surg 2009;197:655-9; discussion: 659.
- Hasen KV, Gallegos ML, Dumanian GA. Extended approach to the vascular pedicle of the gracilis muscle flap: anatomical and clinical study. Plast Reconstr Surg 2003;111:2203-8.
- Graham RG, Omotoso PO, Hudson DA. The effectiveness of muscle flaps for the treatment of prosthetic graft sepsis. Plast Reconstr Surg 2002;109:108-13; discussion: 114-5.
- Illig KA, Alkon JE, Smith A, Rhodes JM, Keefer A, Doyle A, et al. Rotational muscle flap closure for acute groin wound infections following vascular surgery. Ann Vasc Surg 2004;18:661-8.
- Ducic I, Dayan JH, Attinger CE, Curry P. Complex perineal and groin wound reconstruction using the extended dissection technique of the gracilis flap. Plast Reconstr Surg 2008;122:472-8.
- Mayer D, Hasse B, Koelliker J. Long term results of vascular graft and artery preserving treatment with negative pressure wound therpay in Szilagi grade-III infection justify a paradigm shift. Ann Surg 2011;254: 754-60.

3/10/2021
Ahsan T. Ali, MD,a Mario Rueda, MD,b Sarasijhaa Desikan, MD,a Mohammed M. Moursi, MD,a Ruosu An, MD,a Horace Spencer, MS,a Steven Rueda, MD,c and John F. Eidt, MD,d Little Rock, Ark; Baltimore, Md; Cleveland, Ohio; and Dallas, Tex
Published August 2016
Ahsan T. Ali, MD,a Mario Rueda, MD,b Sarasijhaa Desikan, MD,a Mohammed M. Moursi, MD,a Ruosu An, MD,a Horace Spencer, MS,a Steven Rueda, MD,c and John F. Eidt, MD,d Little Rock, Ark; Baltimore, Md; Cleveland, Ohio; and Dallas, Tex
Outcomes after retroflexed gracilis muscle flap for vascular infections in the groin
Summary
Many patients suffer from infections of the groin which require vascular surgery, mainly femoral artery bypass. To make sure that there are no new infections post surgery, GMF(gracilis muscle flap) is an effective option.
From 1997 to 2012, GMF was performed in 68 limbs (64 patients) by vascular surgeons to prevent infections in the common femoral artery.