-
From the Society for Vascular Surgery
Long-term results of the treatment of aortic graft infection by in situ replacement with femoral popliteal vein grafts
Ahsan T. Ali, MD,a J. Gregory Modrall, MD,b Jennie Hocking, PA,b R. James Valentine, MD,b Horace Spencer, PhD,a John F. Eidt, MD,a and G. Patrick Clagett, MD,b Little Rock, Ark; and Dallas, Tex
Objective: Graft excision and neo-aortoiliac system (NAIS) reconstruction with large caliber, femoral popliteal vein (FPV) grafts have been reported as successful treatment of aortic graft infection (AGI) in several small series with limited follow-up. The goal of this study was to evaluate long-term outcomes in large cohort of consecutive patients treated with NAIS for AGI.
Methods: From 1990 to 2006, 187 patients (age: 63 ± 10 years) with AGI were treated with in situ reconstructions using 336 FPV grafts. Data from a prospectively maintained data base were analyzed.
Results: NAIS reconstruction was performed for 144 infected aortofemoral bypasses, 21 infected aortic-iliac grafts, and 22 infected axillofemoral bypasses that had been placed to treat AGI. Polymicrobial cultures were present in 37% while 17% showed no growth. There were 55% gram positive, 32% gram negative, 13% anaerobic, and 18% fungal infections. The mean Society for Vascular Surgery run-off resistance score was 4.5 ± 2.3. Concomitant infrainguinal bypass was necessary in 27 (14%) patients (32 limbs). Major amputations were performed in 14 (7.4%) patients. Out of 14 amputations, five patients had irreversible ischemia and in four, there was no conduit available. Graft disruption from reinfection occurred in 10 patients (5%). While 30-day mortality was 10%, procedure-related mortality was 14%. Independent risk factors for perioperative death on multivariate analysis were: preoperative sepsis (odds ratio [OR] 3.5) ASA class 4 (OR 2.9), Candida species (OR 3.4), Candida glabrata (OR 7.6), Klebsiella pneumoniae (OR 3.5), and Bacteroides fragilis (OR 4.1). Perioperative factors included use of platelets (OR 2.4), blood loss >3.0 liters (OR 9.5). Cumulative primary patency at 72 months was 81%; secondary/assisted primary patency was 91%. Limb salvage at 72 months was 89%. Five-year survival was 52%.
Conclusions: These results compare favorably with other methods of treating AGI, especially in patients with multilevel occlusive disease. Principle advantages include acceptable perioperative mortality, low amputation rate, superior durability with excellent long-term patency, and freedom from secondary interventions and recurrent infections. ( J Vasc Surg 2009;50:30-9.)
Aortic graft infection (AGI) is among the most morbid complications of vascular surgery. The primary treatment objectives are to remove infected graft material and to re-establish vascular continuity with an extra-anatomic bypass or in situ graft replacement. Despite significant progress in perioperative care and antimicrobial therapy, mortality and morbidity remain high.
Graft excision with extra-anatomic bypass was the prevalent treatment in the 1980s and early 1990s and continues to be widely practiced.1-3 More recently, in situ graft replacement is becoming increasingly popular because it is expedient, avoids an aortic stump with the potential for blowout and is associated with a low amputation rate.4,5 A
From the Division of Vascular Surgery, Department of Surgery, University of Arkansas for Medical Sciences, Little Rock;a and the Division of Vascular and Endovascular Surgery, Department of Surgery, University of Texas Southwestern Dallas, Dallas.b
Competition of interest: none
Presented at the annual meeting of the Society for Vascular Surgery, San Diego Calif, June 7, 2008.
Reprint requests: G. Patrick Clagett, MD, Professor and Chairman, Division of Vascular and Endovascular Surgery, Department of Surgery, University of Texas Southwestern at Dallas, Dallas, TX (e-mail: patrick.clagett@ utsouthwestern.edu).
0741-5214/$36.00
Copyright © 2009 by the Society for Vascular Surgery. doi:10.1016/j.jvs.2009.01.008recent meta-analysis from pooled data demonstrated several advantages for in situ graft replacement over extraanatomic bypass.6 There are currently three options for in situ graft replacement for AGI: cryo-preserved allograft, antibiotic soaked synthetic graft, and the autogenous femoral popliteal vein (FPV) graft. In an early series with limited follow-up, Rifampin soaked Dacron was shown to be effective treatment for AGI with low virulent and limited S epidermidis infections.5 Recently, two large series in which AGI was treated with excision and in situ revascularization with either cryo-preserved allograft or Rifampinsoaked Dacron grafts have reported good results.7,8
The third option for in situ replacement is to use femoral popliteal vein grafts. This operation, termed the neo-aortoiliac system (NAIS) procedure represents the only durable autogenous reconstruction. Initially reported by Schulman9-11 as a conduit for femoral popliteal bypass, FPV grafts were first described for AGI treatment in 1993.12 In 1997, an expanded series with moderate follow-up was reported.13 Since then, the NAIS procedure has been widely practiced and reported with similar results in several small series.5,14,15
The purpose of the current study is to provide a comprehensive and robust, long-term follow-up in a large cohort of patients treated for AGI with NAIS reconstruction.
Ali et al 31
JOURNAL OF VASCULAR SURGERY
Volume 50, Number 1
Furthermore, the large number of patients in this series serves to provide a realistic view of morbidity and mortality associated with the procedure. We hypothesize that the NAIS reconstruction is associated with acceptable morbidity and mortality and offers the advantages of superior long-term patency, durability, and freedom of secondary interventions.
METHODS
Patient population. A retrospective review was carried out for all the patients treated for AGIs from November 1990 to December 2006 at two tertiary care university hospitals. All patients had been entered into a prospectively maintained data-base. Dates for inclusion of patients from the University of Arkansas Medical Center were from 2003 to 2006, whereas those from the University of Texas Southwestern Medical Center spanned the entire period and comprised more than 90% of the patients in the series. In order to provide a fully comprehensive review of the NAIS procedure for AGI and to note changes in outcomes over time, the current study includes patients previously reported.12,13,16 The prior reports contained patients undergoing NAIS reconstruction for AGI and other aortic pathology, such as mycotic aneurysm. However, only the patients with AGI were included in the current series. Other operations for treatment of AGI were rarely used and only in unstable patients who could not tolerate the NAIS procedure or in patients who did not have an adequate FPVs. Therefore, this represents a consecutive patient series that includes all patients treated for AGI who were eligible for NAIS reconstruction.
Patient work-up. Noninvasive vascular laboratory evaluation included ankle-brachial indices (ABI). Duplex ultrasound evaluation was also used to assess the quality and diameter of the FPV. A vein less than 6mmin diameter or chronically occluded FPV was not routinely used. Graft duplex imaging has been used as an additional diagnostic tool. The finding of a hypoechoic rim surrounding the graft is compatible with graft infection and, in our experience, has proved useful in the diagnosis.17 Nuclear white cell scans were rarely used. The final diagnosis of AGI was made during surgery and was based on purulent fluid surrounding nonincorporated graft. Early in the series, preoperative workup included catheter arteriography and was later replaced by computed tomographic arteriography (CTA). The arterial outflow was assessed and assigned a run-off score according to the reporting standards on chronic limb ischemia by the Society for Vascular Surgery (SVS).18
Operative technique. The operative technique has been previously published.19 Since 2002, FPV valves have been ablated under direct vision after eversion of the entire length of the harvested vein. This change in technique occurred because valve ablation with valvulotomes was found to be inadequate and believed to be a source for vein graft stenosis requiring intervention that occurred in 4.8% of patients.20 The only other significant technical modification has been staging the operation in a minority of cases in this series (n = 5). This approach entailed dissection of
the FPVs at one setting, followed by vein graft harvest, excision of the infected graft, and NAIS reconstruction one day later, as described by Ali et al.21 In the postoperative period, patients were routinely treated with subcutaneous low dose heparin and calf intermittent pneumatic compression for venous thromboembolism prophylaxis.
Vein grafts were usually oriented in a nonreversed fashion to allow the larger proximal end to be comfortably anastomosed to the aorta. Most proximal anastomoses were end-to-end. However, if the diameter of the aorta greatly exceeded that of the FPV graft, a “pantaloon” configuration was employed whereby two FPV grafts were joined to increase the diameter of the conduit.13 Purulent fluid, grossly infected tissue, thrombus, and graft material were routinely cultured for bacteria and fungi by standard microbiologic techniques.
Data collection and analysis. Charts were reviewed for basic demographics, associated comorbidities, clinical presentation, and culture results. Operative data included total operative time, intravenous fluid, and transfusion requirements and American Society of Anesthesiologists (ASA) patient class. Adjunctive surgical procedures were recorded as either vascular or nonvascular. Vascular procedures included concomitant infrainguinal bypass or a visceral/renal revascularization. Nonvascular procedures included placement of a feeding tube, tracheostomy, muscle flap transfer for coverage in the groin, and duodenal repair or bowel resection.
Patients were divided into three groups according to the location of the infected graft: aortofemoral bypass grafts (AFBG); aortoiliac or aortic tube graft (AIBG); and axillofemoral bypass grafts (AxFBG) grafts. The latter group represented patients who had undergone previous treatment for AGI by AFBG excision and an extra anatomic bypass.
Operative complications were divided into surgical and medical. Surgical complications were specific to the procedure such as limb loss, surgical bleeding, compartment syndrome requiring fasciotomy, mesenteric ischemia, and graft thrombosis. Medical complications included pneumonia, respiratory failure, myocardial infarction, and other complications that might be expected to follow major surgery.
Standard 30-day mortality was recorded. However, because some patients died as a result of the operation later in the hospital course, at a rehabilitation unit or at home, we also assessed procedure-related mortality regardless of the postoperative time span.2 ICU time and total hospital length of stay was recorded. Information was also collected about discharge disposition to home, rehabilitation facility, or to a long-term skilled nursing facility.
The standard follow-up of patients who were geographically close consisted of duplex graft surveillance and clinical examination every 4 months during the first year and then every 6 to 12 months thereafter. If indicated, imaging was obtained using either a conventional arteriogram or a CTA. For out of state patients, follow-up was done through a telephone inquiry to the patients or the
JOURNAL OF VASCULAR SURGERY
July 2009Ali et al
physicians responsible for their care. Social security death index was queried for all the patients who were not actively being followed or lost to follow-up. Primary and secondary patency and limb salvage data were defined according to the reporting standards for the SVS.18 Graft failure was defined as an occluded graft or stenosis requiring intervention. Graft disruption or dehiscence due to nontechnical cause was considered to be due to graft reinfection.
Data were entered into a registry using Microsoft Access (Microsoft, Redwood, Calif). Demographic, clinical, procedural, and postoperative variables were summarized using means and standard deviations for continuous data, or percentages and counts for categorical data. The method of Kaplan-Meier analysis was used to estimate patient survival, graft patency, and limb salvage distributions.22 The log-rank test was used to compare two or more survival distributions.23 Logistic regression was used to model the effect of demographic and medical characteristics on perioperative and 12-month survival. For multivariable logistic regression models, the purposeful selection algorithm described by Hosmer and Lemeshow was used to guide variable selection.24 All statistical analyses were performed using SAS version 9.1 (SAS institute Inc, Cary, NC) or R version 2.6.2 (The R Foundation for Statistical Computing, Vienna, Austria) software.
RESULTS
Demographics. Over a 15-year period, 187 patients were treated for AGI with partial or total removal of the aortic prosthesis and NAIS. There were five patients lost to follow-up after the first postoperative visit. They were not found in the social security death index data base. All other patients had at least a 12-month follow-up. The median follow up was 32 (range 12-168) months. Twelve patients were treated for AGI with either antibiotic-soaked Dacron grafts or extra-anatomic bypass during this period. These patients did not undergo NAIS procedure because of instability from bleeding aortoenteric fistulas or absence of adequate FPVs. Occluded aortic grafts with tissue incorporation and negative cultures were present in 17 patients, and these were considered noninfectious graft failure and therefore not included in the series.
Men comprised the majority (119 [64%]) of patients and the mean age of the entire group was 63 ± 10 years (median age = 64 years) with a range of 29 to 88 years (Table I). Risk factors for atherosclerosis were, as expected, highly prevalent in this population with smoking (97%), hypertension (92%), and coronary artery disease (56%) being the most prominent. Of note, there was use of systemic steroids or other immune suppressive drugs in eight (4%) patients on presentation.Presentation. Out of 187 patients, 140 (75%) underwent original aortic surgery for occlusive disease, 37 (20%) for aneurysmal disease, and 10 (5%) for both. There were 144 (77.4%) AFBG, 21 (11.2%) AIBG, and 22 (11.7%) axillofemoral (AxFem) infections. The predominance of occlusive disease was also reflected in the preoperative (immediately before NAIS operation) ABIs (0.75 ± 0.2 and 0.74 ± 0.3 in the right and left lower extremities, respectively) and the mean SVS run-off scores (4.4 ± 2.1 and 4.6 ± 2.3 on the right and left sides, respectively) (Table I). Twenty two (11.7%) patients had acute limb ischemia on presentation and, of these, five were judged to have irreversible ischemia and nonsalvageable limbs. The SVS run-off score was equal to or higher than six in 47% of patients for the right lower extremity and 48% for the left lower extremity, respectively. Thus, there was a single vessel (usually the profunda femoris artery) available for anastomosis in nearly half of the cases.
The most common presentation was open groin wounds (43%) followed by femoral pseudoaneurysm in 36% (Table I). Graft thrombosis and ischemia were evident in 29%. Aortoenteric erosion/fistula (AEF) was diagnosed in 14% of the patients.
Table I. Patient characteristics
Total (n = 187)
Demographics
-
Age (y) (range)63.2 ± 10 (29-88)
-
Median (25-75 qtr.)64 (56-72)
-
Men119 (63%)
-
Risk factors
-
Smoking179 (96%)
-
Hypertension172 (92%)
-
Coronary disease104 (56%)
-
Hyperlipidemia73 (39%)
-
Diabetes mellitus34 (18%)
-
COPD24 (13%)
-
Renal insufficiency17 (9%)
-
Steroid use8 (4%)
-
End stage renal disease3 (1.7%)
-
Emergency surgery20 (11%)
-
On IV antibiotics81 (43%)
-
Presentation
-
Open groin sinuses81 (43%)
-
Femoral pseudoaneurysm68 (36%)
-
Constitutional61 (32%)
-
Weight loss (19)
-
Ischemia 52 (29%)
-
Sepsis 40 (21%)
-
AE erosion/fistula 26 (14%)
-
Bleeding 23 (12%)
-
Ankle-brachial index
-
Preoperative (right) 0.75 ± 0.2
-
Preoperative (left) 0.74 ± 0.3
-
SVS run-off score
-
Right 4.4 ± 2.1
-
Left 4.6 ± 2.6
-
Infected graft configuration
-
Aortofemoral grafts144 (77%)
-
Aortic tube/iliac grafts21 (11.2%)
-
Failed Ax-fem grafts22 (11.7%)
AE, aortoenteric; AFBG, Aortofemoral bypass grafts; AIBG, aortoiliac or aortic tube grafts; AxFBG, axillofemoral bypass grafts; COPD, chronic obstructive pulmonary disease; IV, Intravenous.
Emergency surgery was defined where the NAIS procedure was done within 24 hours of presentation was necessary in 11% of the patients.
Data reported mean ± SD.Operative data. The median ASA score was 4 with the mean score of 3.6 ± 0.8. Majority (57%) of the patients had
D’Addio et al
JOURNAL OF VASCULAR SURGERY
Volume 50, Number 1
Table II. Operative data
Operative data Total (n = 187)
-
ASA class
-
Median4
-
Mean3.6 ± 0.8
-
Duration (h)9.3± 2.1
-
Aortobifemoral bypass9.5 ± 2.0
-
Aortounifemoral bypass8.1 ± 2.4
-
Aortoiliac bypass7.0 ± 1.1
-
Total number of grafts336
-
Blood loss (L)2.6 ± 3.0
-
Transfusion blood (units)7.5 ± 7.1
-
Transfusion crystalloid (L)6.6 ± 4.2
-
Graft configurations of the NAIS
-
Aortofemoral169 (90%)
-
Aortobifemoral105 (56%)a
-
Aorto-unifem with fem-fem cross over37 (20%)
-
Aortounifemoral27 (14%)
-
Aortoiliac13 (7%)
-
Other5 (2.6%)c
-
Concomitant Fem-pop/Distal bypass (patients)27 (14%)
-
Bypassb
-
(R) 14)
-
(L) 18
-
Total limbs 32
aIncluded proximal anastomosis with a pantaloon graft.
bBilateral bypass was done in five patients.
cAortofemoral or aortoiliac configuration with femoral-popliteal vein (FPV) and great saphenous vein (GSV) composite graft.
an ASA score of 4 or higher. Mean operative time was 9.3± 2.1 hours for the entire group. When both FPVs were utilized, average operating time was 9.5 ± 2.0. Mean operative times for aortounifemoral and aortoiliac NAIS were 8.1 ± 2.4 and 7.0 ± 1.1 hours, respectively. Average operative blood loss was 2.6 liters requiring 7.5 units of blood during surgery. A total of 336 of deep vein grafts were harvested from individual limbs. In three cases, the FPVs were inadequate and the profunda femoris veins were used. Aortofemoral reconstructions with FPVs were performed in the majority of patients (169 or 90%) and these included aortobifemoral (105), aortounifemoral with contralateral femoral crossover bypass (37), and aortounifemoral bypass (no crossover bypass) (27), usually for single limb involvement. Aortoiliac reconstructions with FPVs were performed in only 13 patients (7%). There were five patients where the great saphenous vein (GSV) was used in conjunction with FPV to fashion an iliac or femoral limb of the NAIS (Table II).
Concomitant femoral popliteal or distal bypass was required in 32 limbs of 27 (14%) patients. Right infrainguinal bypass with GSV was performed in 14 limbs and left infrainguinal bypass in 18. Five patients required bilateral lower extremity revascularization. Visceral/renal bypasses were performed in three patients (1.6%). Twenty six patients underwent major duodenal repair or diversion procedures. Forty six patients (25%) had leg fasciotomy (Table III). Most of these were earlier in the series and were prophylactic. The rate of fasciotomy in the past 3 years has declined and is currently 12%.
Table III. Morbidity and mortality
Total (n = 187%)
-
Mortality
-
30-day (n)19 (10%)
-
Procedure related (n)27 (14%)a
-
Discharge disposition
-
Home99 (53%)
-
Rehab facility43 (23%)
-
Nursing home18 (10%)
-
Length of stay
-
(mean)21 ± 8
-
(median) 14
-
Surgical complications
-
Wound complications63 (34%)
-
Fasciotomy46 (25%)
-
Amputations14 (7.4%)a
-
Surgical bleeding10 (5.3%)
-
Mesh closure (abdomen)8 (4.3%)
-
Mesenteric ischemia3 (1.6%)
-
Delayed graft disruptions10 (5.3%)
-
Medical complications
-
Pulmonary 25 (13%)
-
Tracheostomy 17 (9%)
-
Acute renal failure 22 (12%)
-
Coagulopathy 22 (12%)
-
Myocardial infarction 8 (4.3%)
-
Stroke3 (1.6%)
-
Paralysis3 (1.6%)
-
Pulmonary embolism2 (1.1%)
aProcedure related mortality includes death beyond 30 days.
Immediate outcomes. The 30-day mortality was 10% and the procedure-related death rate was 14% (Table III). Details for all deaths are listed in Table IV. The mean length of stay was 21 ± 8 days, however, the median length of stay was 14 days. Out of 187 patients, 27 (14%) did not survive, 99 (53%) were discharged home, 43 (23%) went to a rehabilitation facility and subsequently were discharged home, and 18 (10%) were transferred to a long-term skilled facility.
Limb loss occurred in 14 (7.4%) patients. Five had irreversible ischemia on presentation; four had no autogenous conduit in the presence of grossly infected wounds; two had ligation of the FPV limb as a result of postoperative graft disruption; and one each had limb ischemia from compartment syndrome with extensive myonecrosis, failed distal bypass, and cardiac embolism.
Other complications are listed in Table III. Wound complications occurred in 63 patients (34%). These included all patients who required surgical treatment for groin wounds and vein harvest sites that were treated with muscle flap for coverage, incision and drainage, skin grafting, and intravenous antibiotic therapy. Almost half (44%) of these patients had a purulent groin sinus or septic femoral wounds on presentation. Acute postoperative bleeding occurred in 10 patients (5%) necessitating return to the operating room for control. Two patients had acute postoperative bleeding caused by a slipped tie from one of the FPV grafts.
JOURNAL OF VASCULAR SURGERY
July 2009Ali et al
Table IV. Poor outcomes: Details of 27 procedure related deaths
Age/Sex/Presentation
ASAs
Surgical procedure
Graft microbiology
Complications
Terminal event
75/F AIBG for AAA GI bleed from AEF
4E
Aortoiliac
Candida species Pseudomonas a
Duodenal leak
Died on POD #34 from MSOF
77/F AFBG for occl AEF groin sinuses
4
Aortoiliac, SMA bypass
Staph epi
Mesenteric ischemia, splenic bleeding
Bowel ischemia, fungal sepsis
72/M w AIBG for AAA. AEF w GI bleed
4E
Aortoiliac
Klebsiella pneumoniae, Candida glabrata, Bacteroides f.
Paraplegia, MSOF
Died on POD #27
Died POD #42 MSOF55/F Infect AX-FEM for AFBG infection, retroperitoneal abscess
3
Aortobifemoral
MRSA
Intraoperative cardiac arrest due to bleed
Died POD #2
53/M AFBG sepsis and ketoacidosis
4
Aortobifemoral
Gp B Streptococcus Staph aureus
Lower extremity ischemia
Died on POD #14 from myocardial infarction (autopsy
69/F AFBG and a femfem, graft thrombosis, groin sinus, AEF
4
Aortounifemoral aortoiliac
Candida glabrata, Klebseilla pneumoniae
Duodenal leak
Died on POD #61, graft disruption requiring ligation with amputation
70/M AFGB (AAA) with graft-colonic erosion, on steroids for COPD
4
Aortobifemoral, colectomy,
Candida tropicalis,
Respiratory, renal failure, sepsis
Died on POD #12 from
75/M AFBG (AAA) sepsis, febrile
3
Aortobifemoral
Enterobacter c. MRSE
Possible intraop ligation of splenic vein
Died on POD #6 from splenic rupture (autopsy)
81/M AIBG (AAA) aortic pseudoaneurysm
4
Aortobi iliac,
Salmonella, Yeast, MRSE
Sepsis, pancreatitis,
Died on POD #24 MSOF
69/F AFBG (occl) AEF w bleeding, sepsis
4E
Aortounifemoral, Colon resection,
Klebsiella pneumoniae, E coli Cand albicans,
Perioperative graft thrombosis, bilateral hip disarticulations
Died on POD #48 MSOF
70/F AFBG (AAA) groin sinus
3
Aorotbifemoral
Bacteroides f. Candida species
Groin wound infections
Died on POD #57 from distal graft disruption
61/M AFBG (occl) groin sinuses, active TB
4
Aortobifemoral, pericard window
No data available
Intraop cardiac arrest, Abdomen closure w/ mesh,
Died on POD #24 from MSOF
56/F AFBG/Fem-fem (occl) groin sinus, ischemia
4
Aortounifemoral, fem-fem, fem-pop w GSV
Klebsiella p, E coli, MRSA, Pseudomonas
Compartment syndrome resulting in bilateral amputations, Reinfection
Graft disruption on POD #57 requiring ligation. Died shortly after MSOF
65/F Ax-FEM (occl) groin sinus
4
Aortounifemoral, Fasciotomy
No growth
Mesenteric ischemia from acute SMA thrombosisMesenteric ischemia from acute SMA thrombosis
Died on POD #8 from mesenteric ischemia
73/M AFBG (AAA+occl) AEF w GI bleed, sepsis
4E
Aortobifemoral, Duodenal resection
Candida glabrata, Albicans, Strep Sp.
Wound infections, respiratory failure, coagulopathy
Died on POD #37 MSOF
72/M Aortic (AAA) aortic pseudoaneurysm
4
Aortoiliac, correct midgut volvulus
Candida glabrata, MRSE
ARF, mesenteric ischemia, surgical bleeding
Died on POD #26 from mesenteric ischemia
76/M AFBG (occl) Fem pseudo aneurysm
3
Aortobifemoral, fasciotomy
No growth
Massive surgical bleeding, coagulopathy, Abdomen closure w/mesh
Died on POD #35 MSOF
66/M AFBG (occl) ischemia, groin sinuses
4
Aortobifemoral,
Propionibacter
ARF, respiratory failure, Abd mesh closure
Died on POD #28 from MSOF
76/M AFBG (occl), bleeding femoral pseudoaneurysm, cirrhosis
4E
Aortobifemoral, tracheostomy
Enterobacter, Strep Sp. Bacteroides f.
Coagulopathy, liver failure
Died from coagulopathy on POD #2
70/M AFBG (occl) AEF w GI bleeding, sepsis
4E
Aortofem, fem-fem, Fem-pop w GSV, Fasciotomy, duodenal resection
Candida glabrata, Lactobacillus
Lower extremity ischemia, leak from the duodenal repair
Died on POD #16 from graft disruption w recurrent AEF
66/M AFBG (occl) Graft thrombosis, ischemia
4
Aortobifemoral, splenectomy
Serratia M (2 strains) MRSE
Respiratory failure
Cardiac arrest on POD #12, anoxic brain injury
60/F AFBG (occl), febrile, sepsis
3
Aortobifemoral, bilat. Fem-pop w/GSV, Fasciotomy
Staph epidermidis
Wound infection requiring multiple wound debridments
Died on POD #16 from MSOF, malnutrition
Ali et al
JOURNAL OF VASCULAR SURGERY
Volume 50, Number 1Table IV. Continued
Age/Sex/Presentation
ASAs
Surgical procedure
Graft microbiology
Complications
Terminal event
75/F AFBG, Ax-fem (occl) with groin sinus, ischemia
4
Aorotfemoral, femfem, Subclavian bypass
Klebseilla pneumoniae, Candida glabrata, Enterococcus MRSE
Abdominal sepsis, hematoma, liver failure
Died on POD #23 from sepsis
55/F AFBG (occl) graft thrombosis, ischemia
3
Aortobifemoral
No growth
Surgical bleeding from postop, heparin for mitral valve
Cardiac arrest on POD #15
81/M AIB (AAA) femoral pseudoaneurysm bleeding
4
Aortoiliac
Not available
Pt. had metastatic liver cancer
Died on POD #8 from liver failure #15
79/M AFBG (occl) AEF, sepsis
4E
Aortofem, fem-fem, Duodenal resection
Pseudomonas a., Enterobacter c., Candida albicans
Duodenal leak, sepsis, ARF,
Graft disruption requiring ligation. Died on POD #19 from myocardial infarction
69/F Aortic graft( AAA) Psoas abscess
4
Aortoiliac, splenectomy
Bacteroides f., Enterobacter c.
Graft disruption POD #11, requiring FPV ligation and fem-fem bypass renal, respiratory failure
Died on POD #16 MSOF,
AAA, abdominal aortic aneurysm; AEF, aortoenteric fistula/erosion; AIBG, aortoiliac bypass; ARF, acute renal failure; AXFemBG, axillary-femoral bypass graft; Bacteroides f, Bacteroides Fragilis; E, emergency; E.coli, Escherichia coli; Enterobacter C, Enterobacter Cloacae; F, female; FPV, femoral popliteal vein; GI, gastrointestinal; Gp B, Group B; Klebsiella P, Klebsiella pneumoniae; M, male; MRSE, Methicillin resistant Staphylococcal Epidermidis; MSOF, Multisystem organ failure; Occl, occlusive disease; POD, postoperative day; Pseudomonas a, Pseudomonas Aeruginosa; Pt, Patient; Serratia M, Serratia Marcescens; SMA, superior mesenteric artery; Staph epi, Staphylococcal Epidermidis; Strep Sp, Streptococcal species.
The incidence of graft disruption was 5% (10 patients). There were five proximal (intra-abdominal) disruptions and five distal (femoral) disruptions. These have been previously reported.25 Most of the graft disruptions occurred within the first 2 weeks after NAIS. We attribute this to reinfection but this was documented in only a few of these patients. One patient treated with aggressive immunosuppression had a graft disruption at 7 months caused by recurrent infection surrounding the aortic anastomosis. The majority (6 out of 10) had initial presentation with an AE fistula or erosion. Five out of six had documented duodenal leaks after repair of the duodenum. Some of the patients with graft disruption had severe malnutrition with nonhealing femoral wounds and exposed FPV grafts. All the patients with graft disruptions had polymicrobial infections (Table IV). Only four out of 10 patients survived graft disruption.
The most common medical complication was pulmonary failure requiring ventilator support for extended period of time (Table III). Of these, 17 patients (9%) required a tracheostomy. Acute renal failure occurred in 22 patients (12%) who required temporary hemodialysis. The rate of lower extremity paralysis was 1.6% (three patients) and all of these occurred early in the series.13 There have been no cases of paralysis in the last 140 consecutive patients.Microbiology. There was no information available on seven (4%) patients. Polymicrobial cultures were present in 37% while a single organism was identified in 45% of the cases. Gram positive organisms were most prevalent, 55%. Fungal cultures were present in 17%, and anaerobic infections were present in (14%). There were 17% of patients where no organism was identified on final cultures (Table V).
Long-term outcomes. Patency, limb salvage, and overall survival data are presented in Figs 1, 2, and 3. At 72 months, the primary and assisted/secondary patency were 81% and 91%, respectively (Fig 1). Limb salvage was 89% at 7 years (Fig 2). All amputations
Table V. Microbiology
-
No growth31 (17%)
-
Single organism81 (45%)
-
Multiple organism67 (37%)a
-
No data available7 (4%)
-
Data available180 (96%)
-
Typea43 (23%)
-
Gram positive100 (55%)
-
Gram negative57 (32%)
-
Anaerobes24 (13%)
-
Fungal 32 (18%)
aProcedure related mortality includes death beyond 30 days.aMutually exclusive with more than one type of bacteria was cultured in 37% of the patients
during long-term follow-up occurred because of progression of distal occlusive disease. Overall survival at 5 years was 52% with most patients dying of cardiovascular disease (Fig 3). Patients who presented with AE fistula did worse compared with those without AE fistula for perioperative period and on long term follow-up (log-rank P = .006, Fig 4). Multivariate independent predictors of death or poor out come were reported for the immediate, perioperative period and 12 months after surgery (Table VI). The perioperative factors included microorganism: Candida species with Candida glabrata in particular, Klebsiella pneumoniae, and Bacteroides fragilis infections. Additionally, perioperative sepsis was a predictor of poor
Ali et al
JOURNAL OF VASCULAR SURGERY
July 2009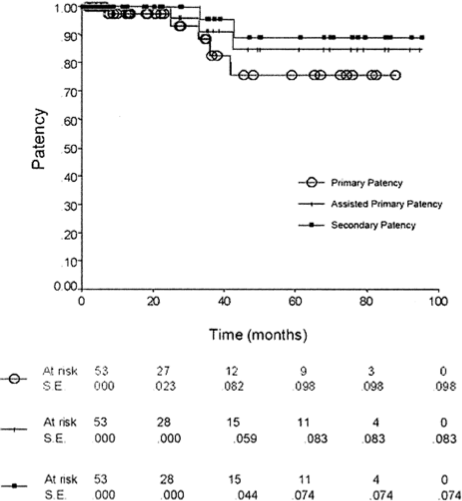
Fig 1. Primary and assisted primary/secondary patency. The patencies is 81% and assisted primary/secondary patency is at 91% with standard error at < 5%. The numbers at risk are noted at each time point.

Fig 2. Limb salvage. Limb salvage rate is 89% at 7 years. The numbers of limbs at risk are noted at each time point. The standard error (in parentheses) at 7 years is 3.1%.
outcome. Operative bleeding was the strongest predictor when the blood loss was greater than 3000 mL, which was probably indicative of the difficulty of the procedure. Factors associated with poor 12-month survival were Age >64 (OR 3.3 P = .005), initial presentation with proximal or distal anastomotic bleeding (OR 2.8 P = .01), chronic renal insufficiency (OR 3.3 P = .004), fungal infection (OR 3.1, P = 0.01) and ASA class 4 or higher (OR 5.3 P < .001) (Table VI). Patients with no growth on culture of graft and purulent material had

Fig 3. Survival. The survival at 5 years is 52.4%. The numbers at risk are noted at each time point. Standard error (in parentheses) at 6 years is 10%.

Fig 4. Survival for patients with aortoenteric (AE) fistula. Survival differed significantly between patients with and without AE fistula (18.4% vs. 56.7%). Standard errors (in parentheses) exceeded 10% at 7 years for patients without AE fistula and all time points for patients with AE fistula.
better outcomes with a 6% perioperative mortality and a 1 year survival of 87%.
DISCUSSION
This study confirms the theoretical advantages of the NAIS procedure for AGI. Autogenous FPV grafts have unique features that include large caliber, infection resistance, and nonthrombogenic surface that make them an ideal conduit for large arterial reconstructions. On longterm follow-up, we have demonstrated a primary and
Ali et al
JOURNAL OF VASCULAR SURGERY
Volume 50, Number 1Table VI. Predictors of mortality
Perioperative
Odds ratio
P value
Organisms (graft)
Cand. Glabrata
7.6
<.01
Klebsiella P
3.5
0.4
B Fragilis
4.1
0.4
Fungal
3.4
0.2
Preoperative sepsis
3.5
0.2
Blood loss > 3000 mL
9.5
<.001
ASA class 4
2.9
0.2
Platelet transfusion
2.4
0.1
12-month
Age >64
3.3
.005
ASA class 4
5.3
<.001
Fungal graft infection
3.1
01
CRI
3.3
.004
Initial presentation with bleeding
2.8
0.1
Preoperative sepsis
2.0
04
CRI, Chronic renal insufficiency.
Fungal etiology was an independent risk factor in addition to Candida glabrata
sisted patency/secondary of 81% and 91% (Fig 1), respectively. These patencies were achieved in patients with multilevel occlusive disease and poor run off without adjunctive, aggressive antithrombotic therapy. Most of our NAIS patients are treated with aspirin as part of general medical treatment for cardiovascular disease. Only a few are treated with vitamin K antagonists or clopidogrel for other specific cardiac or venous indications. Despite AGI with a wide variety of virulent gram-positive, gram-negative and fungal infections, life-long or even long-term antibiotic therapy beyond 4 to 6 weeks has also been found to be unnecessary.
Durability is also realized because FPV grafts have a low incidence of developing vein graft stenosis, most likely because of their large caliber.20 We were initially concerned about the potential for aneurysmal degeneration, but this has not occurred in our NAIS experience. The potential for chronic venous morbidity after removal of FPVs was also feared. However, we have documented minimal disturbance of venous physiology on long-term follow-up and fewer than 15% of limbs had evidence of significant chronic venous
morbidity (CEAP Class C3-C5) on clinic examination at a mean follow-up of 70 months.26,27 In that study, we described two limbs with more advanced venous morbidity. One limb had brawny edema (CEAP Class C4) and one limb with a healed ulceration (CEAP Class C5). To date, those two limbs are the only limbs with CEAP Class C4 or greater venous morbidity in over 400 limbs from which FPV has been harvested for a variety of operations at our institutions. Although early postoperative swelling of harvested limbs is common, swelling typically subsides over the ensuing weeks. It is rare that we need to prescribe compression stockings at late follow-up of patients after FPV harvest. In addition to NAIS procedures for AGI, we and others have demonstrated the beneficial features of this large caliber vein graft in multiple vascular beds.9,28-31
Acute venous morbidity resulting in the need for fasciotomy remains a problem with harvesting FPVs in some patients. In a previous report, we demonstrated that preexisting advanced lower extremity ischemia, concomitant harvest of ipsilateral GSV, and excessive crystalloid administration during operation were independent risk factors for compartment syndrome and the need for fasciotomy.32 The overall rate of fasciotomy in the current series was 25%. This high rate was due to multiple factors. Most of the fasciotomies were prophylactic and not due to the development of compartment syndrome. They were carried out at the end of the NAIS procedure because of asymmetric calf fullness or other subjective findings. Although we generally have a low threshold to perform fasciotomy at our institution, it is the senior author’s opinion that many of the fasciotomies in this series were unnecessary. It is noteworthy that no fasciotomies were performed in the series of NAIS reconstructions for AGI reported by Nevelsteen et al.14,15Other factors that contributed to the high rate of fasciotomy in this series were that most of the patients had occlusive disease (many had advanced ischemia) and that anesthetic techniques rooted in a philosophy of administering large amounts of crystalloid were prevalent earlier in the series. We have recently demonstrated an overall reduction in complications following the NAIS operations by restricting the amount of intravenous crystalloids administered.33 The rate of fasciotomy has declined in recent years to approximately 12%, and this is most likely due to improved anesthetic techniques and more stringent criteria for the performance of fasciotomy
Much has been made of the postulate that the NAIS procedure is long, technically demanding, and may require two teams. At our institution, we perform this operation with a single team that most often consists of the attending surgeon, vascular fellow, a second or third year general surgery resident, and a medical student. As we document in this report, the operation is long taking 7 to 11 hours on average. This could possibly be shortened with two separate surgical teams (one harvesting veins while the other excises the aortic graft); however, we have not found this necessary or feasible in our practice. Ali et al recently reported shortening the NAIS operation by staging FPV harvest.21 The femoral veins are dissected and left in situ and the aortic graft excision and NAIS reconstruction are carried out 24 hours later.
As far as the technical demands, they are well within the capability of a well-trained vascular surgeon. Throughout our experience, we have noted no “learning curve” in performing this operation. It is noteworthy that our overall mortality has not decreased over time. In fact, it has actually increased. In our previous report, the mortality for NAIS for AGI was 8% and currently it is 10% for 30-day mortality and 14% for procedure-related mortality. This change most likely reflects the more realistic appraisal of mortality that is realized with a larger, more robust series. In addition, we have subjectively noted that referral of sicker patients has
Ali et al
JOURNAL OF VASCULAR SURGERY
July 2009accompanied the reputation garnered by our institution in treating these patients.
The identification of independent risk factors for death after NAIS is of interest. Some are obvious such as massive blood loss, renal failure, high ASA score, and preoperative sepsis (Table VI). Infections with Candida glabrata, Klebsiella pneumoniae, and Bacteroides fragalis are more puzzling. Others have noted poor outcomes with Klebsiella infections as well.8 Anaerobic infections have may be more deadly because these organisms were usually part of polymicrobial infections that are frequently associated with sepsis, debilitation and severe systemic toxicity. The same is true for Candida glabrata infections. Patients with Candida infections in our series generally had a prolonged course of medical therapy with multiple antibiotics prior to referral and suffered from chronic sepsis, malnutrition, and debilitation. Candida glabrata was also frequently found in patients with AE fistula, which was associated with a poor outcome (Fig 4). Recurrent or persistent infection was judged to have been the etiology in the graft disruptions that occurred in 5% of the patients in this series. This is a severe problem in that two-thirds of the patients with graft disruption died from this complication. This is disappointing but not surprising. We have previously reported that graft disruption of FPV grafts occurs in the setting of ongoing contamination of the graft, severe malnutrition, immunosuppression and debilitated state.25 In the 10 patients with disruption, six had AE fistula or erosion. Five of these six patients had had persistent leakage of enteric contents that was contiguous with FPV grafts. Severity of infection is also a contributing factor since all patients with graft disruption had polymicrobial infections. Although FPV grafts are infection resistant, this benefit is realized in patients with intact host defenses and in which there is only temporary contamination of the bed where the vein graft is placed.
Patients with AE fistula represent a subset with especially poor outcomes, regardless of the surgical approach. In the current study, patients with an AE fistula had a higher mortality rate after NAIS reconstruction than patients without an AE fistula (Fig 4). Our group recently reported our experience with AE fistulas using a variety of approaches, including extra-anatomic reconstruction with aortic stump ligation and NAIS reconstruction.34 There was no difference in mortality based on the surgical approach. In that series, the only independent predictors of mortality were age and gastrointestinal complications. As noted in the current study, five of six patients with AE fistulas who experienced graft disruption had duodenal leaks that bathed the vein graft in gastrointestinal luminal contents. This experience provides further evidence that the integrity of the duodenal reconstruction is the primary determinant of outcome, rather than the type of reconstruction or conduit. To minimize the risk of a duodenal leak, we now obtain the assistance of our gastrointestinal surgeons for multilayer closure of the duodenum and selective duodenal diversion. Whenever feasible, we utilizeomentum for meticulous exclusion of the duodenal repair from the NAIS reconstruction or aortic stump. We currently advocate selecting the type of reconstruction for AE fistulas based on several factors, including the stability of the patient, the length of the aortic stump, and our confidence in the duodenal reconstruction. A NAIS reconstruction is not a reasonable option for unstable patients, but may be the most appropriate reconstruction if there is insufficient length of infrarenal aorta for a multilayered aortic stump closure.
We previously reported discontinuing antibiotics 5 to 7 days after NAIS for AGI but we now recommend continuing antibiotic therapy for 4 to 6 weeks in patients with anaerobic, gram negative, and fungal infections. In patients with these infections who are severely malnourished, debilitated, and immunosuppressed, we will consider alternative procedures such as extra-anatomic bypass.
In our series of NAIS reconstructions, the outcome for patients with no growth on culture of the excised graft was better than the larger cohort, including perioperative mortality of 6% and 1-year survival of 87%. We attribute the improved outcomes for this group to probable infection with low virulence organisms that did not grow on standard culture techniques. Others have advocated using antibiotic-soaked Dacron grafts for selected patients with limited, low virulence infections. This is clearly a more expedient operation and has reported low short-term mortality and amputation rate. However, the uncertain risk of reinfection and the requirement for life-long antibiotic therapy limit the usefulness of this approach. The series reporting treating AGI with antibiotic-soaked Dacron grafts are not comparable to ours because they are small, have limited follow-up and have a relative paucity of patients with infected AFBGs who have multilevel occlusive disease with poor run off.4,5,8 For example, in the report from the Mayo clinic, less than half of the patients presented with infected AFBGs.8 Most had limited infections of low virulence with minimal, purulent contamination. We agree that antibiotic-soaked Dacron grafts have a role in the management of AGI and use this in highly selected who are too unstable to undergo NAIS reconstruction. This is usually in the setting of massive gastrointestinal bleeding from aortoenteric fistula. The 50% survival of patients at 5 years who were cured of AGI (Fig 2) is sobering. When presented with a patient with a limited life span, we now consider this option.
For the majority of patients with AGI, we continue to advocate the NAIS reconstruction because of its superior durability in comparison to other procedures. These patients do not require lifelong antibiotic therapy. They also have an excellent outlook for limb salvage. The risk of reinfection is low, as is the need for reintervention for vein graft stenosis.
The authors would like to extent gratitude towards the following individuals: Eva Scoggins, RVT, Julie Fowler, Victor D’Addio, MD, Christopher Bell, MD,
Ali et al
JOURNAL OF VASCULAR SURGERY
Volume 50, Number 1and John Brawley, MD. Without their, help this manuscript would not have been possible.
AUTHOR CONTRIBUTIONS
Conception and design: AA, GC, GM
Analysis and interpretation: AA, GC, GM, JV, JE
Data collection: AA, JH, GC
Writing the article: AA, GC, GM, JV, JE
Critical revision of the article: AA, GC, GM
Final approval of the article: AA, GC
Statistical analysis: AA, HS
Obtained funding: GC
Overall responsibility: GC
REFERENCES
1. Reilly LM, Stoney RJ, Goldstone J, Ehrenfeld WK. Improved management of aortic graft infection: the influence of operation sequence and staging. J Vasc Surg 1987;5:421-31.
2. Seeger JM, Pretus HA, Welborn MB, Ozaki CK, Flynn TC, et al. Long-term outcome after treatment of aortic graft infection with staged extra-anatomic bypass grafting and aortic graft removal. J Vasc Surg 2000;32:451-61.
3. Yeager RA, Taylor LM, Moneta GL, Edwards JM, et al. Improved results with conventional management of infrarenal aortic infections. J Vasc Surg 1999;30:76-83.
4. Bandyk DF, Edward VK, Thomas IR, Holly K, Jonathan BT. Treatment of bacteria-biofilm graft infection by in situ replacement in normal and immune-deficient states. J Vasc Surg 1993;18:398.
5. Bandyk DF, Novotney ML, Back MR, Johnson BL, Schmacht DC. Expanded application of in situ replacement for prosthetic graft infection. J Vasc Surg 2001;34:411.
6. O’Connor S, Andrew P, Batt M, Becquemin JP. A systematic review and meta-analysis of treatments for aortic graft infection. J Vasc Surg 2006; 44:38.
7. Kieffer E, Gomes D, Chiche L, Fleron M-H, Koskas F, Bahnini A. Allograft replacement for infrarenal aortic graft infection: early and late results in 179 patients. J Vasc Surg 2004;39:1009.
8. Oderich GS, Bower TC, Cherry KJ, Panneton JM, Sullivan TM, Noel AA, et al. Evolution from axillofemoral to in situ prosthetic reconstruction for the treatment of aortic graft infections at a single center. J Vasc Surg 2006;43:1166.
9. Schulman ML, Badhey MR, Yatco R. Superficial femoral popliteal veins and reversed saphenous veins as primary femoropopliteal bypass grafts: a randomized comparative study. J Vasc Surg 1987;6:1.
10. Schulman ML, Badhey MR, Yatco R, Pillari G. An 11-year experience with deep leg veins as femoropopliteal bypass grafts. Arch Surg 1986; 121:1010.
11. Schulman ML, Schulman LG. Deep leg veins as femoropopliteal bypass grafts. World J Surg 1990;14:843.
12. Clagett GP, Bowers BL, Lopez-Viego MA, Rossi MB, Valentine RJ, Myers SI, et al. Creation of a neo-aortoiliac system from lower extremity deep and superficial veins. Ann Surg 1993;218:239-48; discussion 239-48.
13. Clagett GP, Valentine RJ, Hagino RT. Autogenous aortoiliac/femoral reconstruction from superficial femoral popliteal veins: feasibility and durability. J Vasc Surg 1997;25:255-66; discussion 267-70.
14. Nevelsteen A, Lacroix H, Suy R. Autogenous reconstruction with the lower extremity deep veins: an alternative treatment of prosthetic infection after reconstructive surgery for aortoiliac disease. J Vasc Surg 1995;22:129.
15. Nevelsteen A, Lacroix H, Suy R. Infrarenal aortic graft infection: in situ aortoiliofemoral reconstruction with the lower extremity deep veins. Eur J Vasc Endovasc Surg 1997;14:88.16. Gordan LL, Hagino RT, Jackson MR, Modrall JG, Valentine RJ, Clagett GP. Complex aortofemoral prosthetic infections: the role of autogenous superficial femoropopliteal vein reconstruction. Arch Surg 1999;134:615-21.
17. DeMuth RP, Kaylor C, Serrano S, Arko FA, Valentine RJ, Clagett GP. Hypoacoustic luminal outline (halo); an adjunct diagnostic ultrasound feature of late graft infection. J Vasc Ultrasound (in press).
18. Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia. J Vasc Surg 1997;26:517-38.
19. D’Addio VJ, Clagett G. Surgical treatment of the infected aortic graft. ACS Surgery, principles and practice. New York: WebMD, 2005.
20. Beck AW, Erin HM, Hocking JA, Timaran C, Arko F, Clagett PG. Aortic reconstruction with femoral popliteal vein: graft stenosis incidence, risk and reintervention. J Vasc Surg 2008;47:36.
21. Ali A, Mcleod N, Kalapatpau VR, Moursi MM, Eidt JF. Staging the neoaortioiliac system: feasibility and short-term outcomes. J Vasc Surg 2008;48:1125-32.
22. Kaplan E, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc 1958;53:457-81.
23. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966;50:163-70.
24. Hosmer DL. Applied logistic regression. Hoboken, NJ: John Wiley & Sons Inc, 2000.
25. Ali A, Bell C, Modrall JG, Valentine RJ, Clagett G. Graft-associated hemorrhage from femoral popliteal vein grafts. J Vasc Surg 2005;42: 667-72.
26. Modrall JG, Hocking JA, Timaran CH, Rosero EB, Arko Iii FR, Valentine RJ, et al. Late incidence of chronic venous insufficiency after deep vein harvest. J Vasc Surg 2007;46:520-5.
27. Wells JK, Hagino RT, Bargmann KM, Jackson MR, Valentine RJ, Kakish HB, et al. Venous morbidity after superficial femoral popliteal vein harvest. J Vasc Surg 1999;29:282.
28. Ali A, Clagett GP, Edwards MJ. Complex venous and arterial reconstruction with deep vein after pelvis exenterative surgery: a case report. Am Surg 2006;72:22-4.
29. Hagino RT, Bengtson TD, Fosdick DA, Valentine RJ, Clagett GP. Venous reconstructions using the superficial femoral popliteal vein. J Vasc Surg 1997;26:829-37.
30. Modrall JG, Joiner DR, Seidel SA, Jackson MR, Valentine RJ, Clagett G. Superficial femoral popliteal vein as a conduit for brachiocephalic arterial reconstructions. Ann Vasc Surg 2002;16:17-23.
31. Modrall JG, Sadjadi J, Joiner DR, Ali A, Welborn MB, Jackson MR, et al. Comparison of superficial femoral vein and saphenous vein as conduits for mesenteric arterial bypass. J Vasc Surg 2003;37:362.
32. Modrall JG, Sadjadi J, Ali AT, Anthony T, Welborn MB, Valentine RJ, Hynan LS, et al. Deep vein harvest: predicting need for fasciotomy. J Vasc Surg 2004;39:387.
33. Adesanya A, Rosero E, Timaran C, Clagett GP, Johnston WE. Intraoperative fluid restriction predicts improved in major vascular surgery. Vasc Endovasc Surg 2009;42:531-6.
34. Valentine R, Timaran CH, Modrall GJ, Smith ST, Arko FR, Clagett GP. Secondary aortoenteric fistulas versus paraprosthetic erosions: is bleeding associated with worse outcome? J Am Coll Surg 2008;207:922-7.
Submitted Oct 13, 2008; accepted Jan 3, 2009.
-
3/10/2021
Ahsan T. Ali, MD,a J. Gregory Modrall, MD,b Jennie Hocking, PA,b R. James Valentine, MD,b Horace Spencer, PhD,a John F. Eidt, MD,a and G. Patrick Clagett, MD,b Little Rock, Ark; and Dallas, Tex
Published July 2009
Ahsan T. Ali, MD,a J. Gregory Modrall, MD,b Jennie Hocking, PA,b R. James Valentine, MD,b Horace Spencer, PhD,a John F. Eidt, MD,a and G. Patrick Clagett, MD,b Little Rock, Ark; and Dallas, Tex
Long term results of the treatment of aortic graft infection by in situ replacement with femoral popliteal vein grafts
Summary
Graft excision and neo-aortoiliac system (NAIS) reconstruction is the best treatment for aortic graft infection (AGI). A study of 16 years from 1990 to 2006, for all the patients treated for AGIs was done.
(NAIS) reconstruction is the better than other procedures because these patients do not require lifelong antibiotic therapy, the risk of reinfection is low, as is the need for reintervention for vein graft stenosis.